In the second quarter of this year. April, ESTEC, held the “Analytical Solutions for Today’s Pharma Industry” Seminar with the aim of training attendees on meeting the Pharma regulatory requirements.
During the seminar, ESTEC aimed to provide comprehensive training to attendees in the pharmaceutical industry, focusing on meeting critical regulatory requirements such as FDA, USP-NF, Ph. Eur, and JP. The event, titled “Analytical Solutions for Today’s Pharma Industry,” covered a diverse range of topics to enhance participants’ understanding and skills in the pharmaceutical analytical field.

Seminar Highlights:
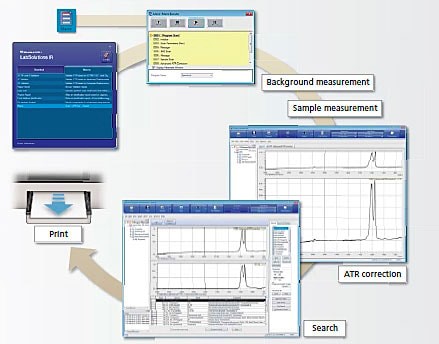
Server-Based Total Solution Software (LIMS): The seminar delved into the implementation of cutting-edge server-based total solution software, particularly LabSolution CS. This software is designed to streamline and optimize the processes involved in pharmaceutical testing instruments, providing a centralized platform for data management.
Technological Advancements in Pharmaceutical Physical Parameters Testing: Attendees had the opportunity to explore the latest technological advancements in testing pharmaceutical physical parameters. The discussion included breakthroughs in analytical instrumentation and techniques, ensuring that participants were up-to-date with the industry’s technological landscape.

Managing Impurities & Cleaning Validation Solutions for Pharma: The seminar addressed crucial aspects of pharmaceutical manufacturing, focusing on managing impurities and implementing effective cleaning validation solutions. This session provided valuable insights into maintaining compliance with industry standards and ensuring the safety and efficacy of pharmaceutical products.
Redefining Separation, Resolution & Throughput in Pharma Testing: The event also explored innovations in separation techniques, resolution enhancement, and throughput optimization in pharmaceutical testing. This segment aimed to redefine the analytical processes involved in pharmaceutical testing, contributing to improved efficiency and accuracy.

Collaboration with Shimadzu Middle East & Africa FZE:
ESTEC collaborated with experts from Shimadzu Middle East & Africa FZE to ensure the delivery of top-notch knowledge from some of the world’s best experts in analytical solutions. Shimadzu, a renowned leader in analytical instrumentation, brought its wealth of experience and expertise to enrich the seminar sessions.

Attendee Experience:
Attendees in the pharmaceutical industry had a valuable experience of learning, engaging in discussions, networking with industry experts, and enjoying the collaborative atmosphere of the event. The seminar provided a platform for professionals to stay abreast of industry trends, exchange ideas, and build meaningful connections within the pharmaceutical analytical community.
This seminar not only contributed to the professional development of the attendees but also fostered a sense of community and collaboration within the pharmaceutical industry. Participants left with enhanced knowledge, practical insights, and a network of contacts to support their ongoing work in the dynamic field of pharmaceutical analytics.




After the successful pharmaceutical seminar, the collaborative teams of ESTEC and SMEA ventured to the Hilton Garden Inn in Kampala, Uganda, strategically extending their reach to the Ugandan market. The ensuing “Stretching Sensitivity & Accuracy Limits in Laboratory Testing” Seminar drew participants from a spectrum of sectors, including Pharmaceuticals, Food & Beverages, Security, Regulatory Bodies, and Education & Research.


The comprehensive seminar agenda covered an array of pertinent topics designed to offer innovative solutions to diverse industries. Discussions delved into the technological advancements in chromatography, encompassing HPLC, LCMCMC, and GCMSMS. Additionally, there was a focus on advancements in physical parameters testing, server-based network software solutions, UTM application, elemental impurities & heavy metal testing solutions, regulatory requirements & accreditation processes, and nitrogen determination & fiber analysis techniques.

The purposeful inclusion of these topics addressed specific needs across sectors such as pharmaceuticals, environmental studies, research initiatives, food & beverages production, steel industries, forensic & security concerns. Attendees not only engaged in insightful discussions but also seized the opportunity to network with professionals from various fields. The event provided a platform for knowledge exchange, skill enhancement, and enjoyable interactions, complemented by delectable food to create a well-rounded and enriching experience for all participants.













 Liquid chromatography (LC) separates the components of a sample based on the differences in their affinity/retention strength during the stationary phase and mobile phase. Common LC techniques, namely reversed-phase, normal phase, and size exclusion chromatography. With technological advancements the LC has evolved to analyze smaller particle sizes and higher pressure that are more efficient, have higher speed, sensitivity, and resolution. This includes the high-performance liquid chromatography (HPLC) and the ultrahigh-performance liquid chromatography (UHPLC).
Liquid chromatography (LC) separates the components of a sample based on the differences in their affinity/retention strength during the stationary phase and mobile phase. Common LC techniques, namely reversed-phase, normal phase, and size exclusion chromatography. With technological advancements the LC has evolved to analyze smaller particle sizes and higher pressure that are more efficient, have higher speed, sensitivity, and resolution. This includes the high-performance liquid chromatography (HPLC) and the ultrahigh-performance liquid chromatography (UHPLC).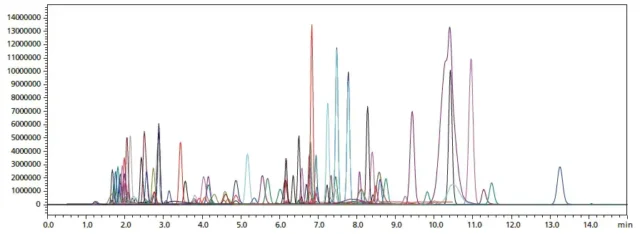 Source
Source
























