As we kick off 2026, I’m feeling a bit reflective. I’m looking back at how far I’ve grown—and I don’t just mean “maturing” or the inevitable addition of “holiday weight” that seems to defy the laws of thermodynamics. I’m talking about professional evolution.
I’ve realized I’m a dangerous mix of someone who loves listening to stories and yet can’t stop telling them. So, grab a lab-grade coffee, and let’s travel back to 2012. It feels like a lifetime ago, doesn’t it?
The “Character Development” Era
Joining Cosmos Limited as a young Laboratory Analyst was a literal dream come true. Mostly because I had done enough job hunting for three lifetimes and my shoes were starting to give up on me. I walked into that lab ready to conquer the world, but instead, I got what the kids these days call “character development.” (If you want the spicy details of that specific trauma, I’ve linked the blog post below!)
What blew my mind back then was my first encounter with a Laboratory Information Management System (LIMS). To me, it was pure sorcery. You configured the products, and boom—you were ready. Samples would roll in from Production, Stability, R&D, and Process Validation like a never-ending tide. A designated analyst would log them in, a Section In-Charge would play “Sample Tetris” to allocate tests, and then there was me.
My entire life lived on a printed worksheet. I’d log in, print my “Official Paperwork,” carry out the tests, and then manually feed the system every weight and dilution I’d performed. It felt high-tech at the time, seeing a system-generated Certificate of Analysis (CoA). I thought I had reached the peak of pharmaceutical science.
I was wrong. Very, very wrong.
The “WHO-Preach” Standard
Then came the transition to MEDS. If Cosmos gave me “character development,” MEDS gave me a reality check so hard it left a dent.
This lab was WHO-prequalified. The standards were so high I felt like I needed permission just to breathe near the glassware. I’m not trying to be a walking billboard for them, but waaaaah—the level of discipline was on another planet.
When I first joined, there was no LIMS. However, the Quality Assurance Manager (who shall remain nameless to protect the visionary) had a dream. He used to talk about “Global Platforms” and “International Standards” while we were still knee-deep in paperwork. To be honest? I was the Doubting Thomas of the lab. I’d nod and smile, thinking, “Sure, and maybe one day the HPLCs will make me a sandwich too.”
The Paperless Revolution
But then, it happened. The vision became a reality. We went paperless.
It wasn’t just a system; it was a living, breathing digital ecosystem. It was linked to everything. Data was captured in real-time, fulfilling every letter of ALCOA+ (Data Integrity) without us even trying. You could request inventory, deduct reagents, and monitor stock levels online. The methods of analysis were right there on the server, accessible from any computer. No more frantic hunting for a stained, dog-eared SOP folder while an auditor breathed down your neck.
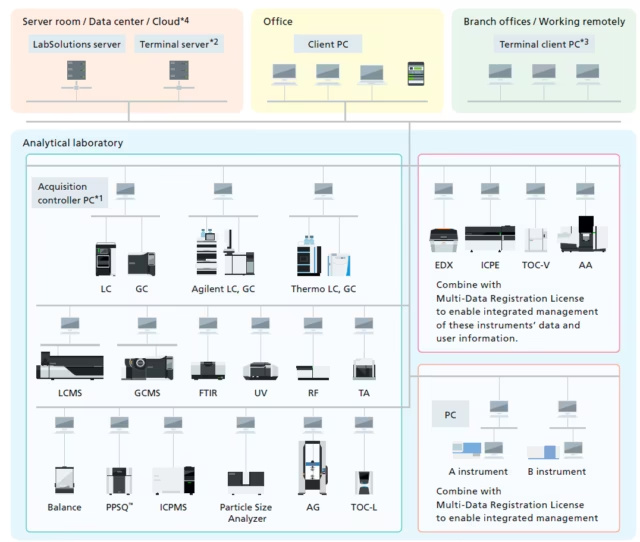
The Secret Sauce: Shimadzu LabSolutions CS
Now, here is the “Aha!” moment of my story. The reason that system ran like a well-oiled Ferrari wasn’t just luck—it was Shimadzu LabSolutions CS.
Before LabSolutions CS, a supervisor’s job involved a lot of “Lab Aerobics”—walking from bench to bench, checking screens, and hunting for results. With LabSolutions CS, the game changed:
- The “Comfort” Factor: Supervisors can review data and monitor the performance of HPLCs, Spectrometers, and even Balances from the comfort of their office. It’s like having a CCTV feed for your data, minus the creepiness.
- Resource Management: It tracks exactly how long each piece of equipment has been running. No more “guessing” which HPLC is the workhorse and which one is just sitting there looking pretty.
- Centralized Control: All the data lives in one secure, centralized vault. No more “I think I saved it on the D: drive of PC #4.”
The Full Circle
Here is the hilarious and beautiful part: I am currently back at Cosmos Limited—the very place where I started my journey and grew my first ‘grey hairs’. But this time, I’m not the girl hunting for a job.
I am here helping to install and provide application support for LabSolutions CS.
It’s a total full-circle moment. I’m giving back to the company that jumpstarted my career by equipping them with the tools to ensure absolute data integrity and peak efficiency. We’re moving from the “Character Development” manual days to the “Global Standard” digital era.
As I look at these Shimadzu systems being configured, I can’t help but laugh. 2012-me wouldn’t believe it. But 2026-me knows: with the right vision (and the right software), the lab doesn’t have to be a place of chaos—it can be a place of pure, automated inspiration.
Written By: Claire Ogeto – Application Specialist



 Mitchelle
Mitchelle  Tom
Tom Sailesh Dhamale, Training Manager, SMEA
Sailesh Dhamale, Training Manager, SMEA Anant, LC Specialist, SMEA
Anant, LC Specialist, SMEA Sampath, Spectroscopy Specialist, SMEA
Sampath, Spectroscopy Specialist, SMEA






















